We take pride in being experts in energy storage solutions. This is why we chose a superior battery chemistry that has been proven through decades of research and deployment in multiple applications. In addition, the energy storage solution chosen has numerous advantages over current lead acid technology.
Capacity & Cycle Life
All lithium chemistries have higher energy density compared to lead acid batteries. We use lithium-ion technology because of the dramatic increase in energy density over current lead acid battery solutions. We chose lithium iron phosphate (LiFePO4) because it has a specific energy of ~110 watt-hours per kilogram, compared to lead acids ~40 watt-hours per kilogram. What does this mean? Our batteries can be ~1/3 the weight for similar amp-hour ratings.
Not only do lithium-ion battery packs store more energy, but the cycle-lifetime far exceeds that of lead acid and many other lithium chemistries.
Every battery cell chemistry is affected by the depth of discharge, and the deeper the discharge, the shorter the lifespan. Our lithium-ion can be discharged to 80% while still maintaining long cycle lifetimes (>3000 cycles). Lead acid batteries experience drastic reductions in cycle life. In fact, at an 80% depth of discharge, lead acid batteries only last 1000-1500 cycles, meaning our batteries last 3x longer.
Speed & Efficiency
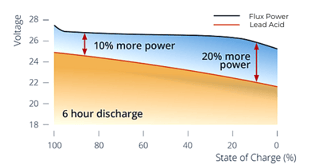
Our lithium-ion batteries are fast. They can be fast-charged completely and can handle ultra-fast charging up to 1C (a full charge in 1 hour). Lead acid can only be fast-charged up to 80% after which the charging current drops dramatically. In addition, our battery packs maintain excellent performance under discharge rates as high as 3C continuous (full discharge in 1/3 an hour) or 5C pulsed. Lead acid experiences dramatic voltage sag and capacity reduction by comparison. In fact, the discharge profile of a lithium-ion battery shows how voltage and power remain almost constant throughout its discharge, unlike lead acid. This means that even when the battery runs low, performance stays high.
There are also no memory issues, discharge and charge the battery at any point without consequence. With lead acid, failure to fully charge leads to sulfation which damages the batteries. This also occurs when storing lead acid while not fully charged. With our lithium-ion, store the battery pack at any state of charge except near zero.
Safety & Reliability
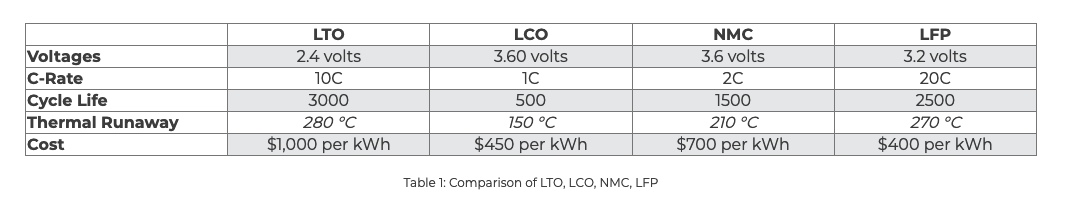
There are a wide variety of chemistries to choose from when looking at advanced lithium batteries. We chose lithium iron phosphate (LiFePO4) because it has three advantages that make it the obvious choice for tough jobs.
-
It is thermally stable up to very high temperatures, meaning no thermal runaway. The batteries can be used safely in ambient temperatures up to 55°C (131°F). Operating lead acid batteries at this temperature reduces their cycle life by a whopping 80%.
-
Lithium iron phosphate provides a remarkably long cycle life, with competing chemistries being either too expensive (lithium titanate), or too unstable (lithium nickel cobalt aluminum oxide).
-
Lithium iron phosphate provides more power and more energy density than lead acid and many other lithium chemistries, so it’s perfect for demanding jobs, and efficient energy storage solutions.
Not all lithium batteries are created equal. There are several factors that go into creating a battery that is high-performing, long-lasting, and most importantly, safe. One major factor to consider is UL certification, or at a minimum making sure the battery is designed to UL standards.
Did you know lithium-ion battery packs are maintenance-free? No electrolyte must be added and there is no danger of acid spills or dangerous vapors. In our case studies, fewer batteries were needed when compared to lead acid, so no storage room is required.
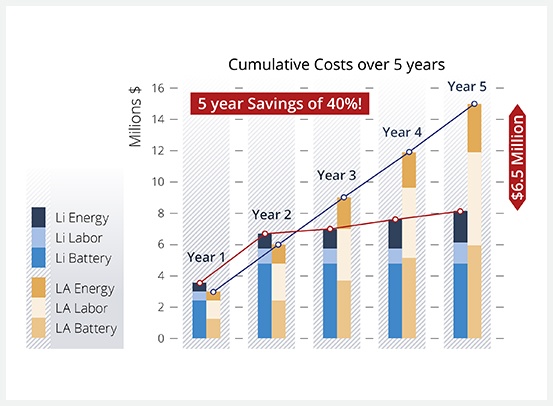
Five Year Savings with Lithium-ion Batteries
Definitions
Ergonomic Risk: Situations that may present risks to people. These include any physical wear and tear on the body or injury related accidents.
UL Recognized: UL Recognized does not apply approval for complete products. Instead, it focuses on components and parts that are used within other products. It certifies that a component within a larger mechanism meets UL standards. UL Recognized is easier to attain than UL Listed.
Class 1 Forklift: Also known as electric motor ride forklifts, Class 1 forklifts can be stand up or sit-down models. These forklifts can include counterbalanced or three-wheel trucks. These forklifts can handle a capacity of 8,000 lbs. or more, making them essential when lifting heavy materials throughout a facility.
Class 2 Forklift: These forklifts are used for multiple applications and can include order pickers, turret trucks, narrow aisle forklifts and more. Many of these forklifts are designed to operate in tight spaces and narrow aisles.
Class 3 Forklift: These forklifts include pallet jacks, walkie stackers, end riders and center riders. Class 3 forklifts are designed to lift loads a few inches off the ground for transportation. They have minimal lift capabilities (i.e. lifting a pallet off the ground) used to transport materials throughout a facility.
Current Rating: The maximum current that a fuse will hold for an amount of time without degrading the fuse.
Thermal Stability: Stability of a fluid and its ability to resist breaking down under heat stress. If the heat reaches max temperatures, the fluid will deteriorate.
SEI Film: SEI film (solid electrolyte interphase) is a layer that is formed from the decomposition or breaking up of the electrolyte of the battery. This is important for lithium-ion batteries because it affects the cycle life.
Internal Resistance: Internal resistance is the resistance in a battery which causes a drop in the source voltage when there is a current. Internal resistance restricts the voltage delivery and determines the battery’s runtime.

Related Content